Fichye:Electrolysis.svg

Taille de cet aperçu PNG pour ce fichier SVG : 624 × 599 piksèl. Lòt rezolisyon yo: 250 × 240 piksèl | 500 × 480 piksèl | 800 × 768 piksèl | 1 067 × 1 024 piksèl | 2 133 × 2 048 piksèl | 976 × 937 piksèl.
Fichye orijinal (Fichye SVG, rezolisyon de 976 × 937 piksèl, gwosè fichye : 42 kio)
Istorik fichye a
Klike sou yon dat/yon lè pou wè fichye a jan li te ye nan moman sa a.
| Dat ak lè | Minyati | Grandè yo | Itilizatè | Komantè | |
|---|---|---|---|---|---|
| Kounye a | 7 mas 2015 à 10:00 |  | 976 × 937 (42 kio) | Goran tek-en | 0(zero) to O (capital O) |
| 20 out 2011 à 09:48 | 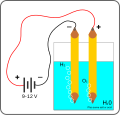 | 976 × 937 (50 kio) | Nevit | border | |
| 20 out 2011 à 09:27 |  | 872 × 819 (46 kio) | Nevit | txt 2 path | |
| 20 out 2011 à 09:25 |  | 872 × 819 (32 kio) | Nevit | fixes | |
| 20 jiyè 2010 à 19:48 |  | 872 × 819 (32 kio) | Nevit | {{Information |Description=Basic setup for demonstration of electrolysis of water at home. |Source={{own}} |Date=2010 07 |Author=Nevit |Permission= |other_versions= }} Category:Electrolysis Category:Nevit Dilmen SVG |
Itilizasyon fichye sa a
paj sa a itilize fichye sa a:
Itilizasyon global fichye a
Wiki sa a yo sèvi ak fichye sa a:
- Itilizasyon sou ar.wikipedia.org
- Itilizasyon sou ca.wikipedia.org
- Itilizasyon sou de.wikipedia.org
- Itilizasyon sou en.wikipedia.org
- Itilizasyon sou en.wikibooks.org
- Itilizasyon sou es.wikipedia.org
- Itilizasyon sou fa.wikipedia.org
- Itilizasyon sou fr.wikipedia.org
- Itilizasyon sou hi.wikipedia.org
- Itilizasyon sou hr.wikipedia.org
- Itilizasyon sou id.wikipedia.org
- Itilizasyon sou kn.wikipedia.org
- Itilizasyon sou pa.wikipedia.org
- Itilizasyon sou ro.wikipedia.org
- Itilizasyon sou sh.wikipedia.org
- Itilizasyon sou ta.wiktionary.org
- Itilizasyon sou th.wikipedia.org
- Itilizasyon sou uz.wikipedia.org
- Itilizasyon sou vi.wikipedia.org
- Itilizasyon sou zh-yue.wikipedia.org
- Itilizasyon sou zh.wikipedia.org


